How viral infection could cause an autoimmune disease
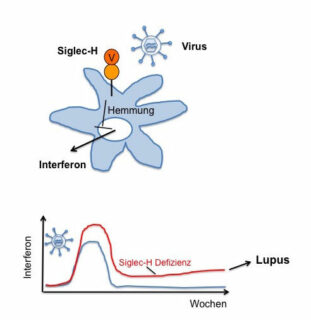
Viral infections may be involved in the pathogenesis of the lupus autoimmune disease. This was recently discovered by FAU researchers. Their research shows that messenger substances of the immune system trigger the disease when their production is not lowered after recovery from viral infection. The findings could open a new long-term approach to therapy for autoimmune diseases.
The autoimmune disease systemic lupus erythematosus (SLE) – also known as lupus – causes the skin, kidneys and blood vessels to be attacked by the body’s own immune system. Left untreated, the disease can at worst lead to multiple organ failure. Researchers at FAU have now been able to decipher another puzzle piece in understanding how this autoimmune disease is triggered.
Using a mouse model, the researchers Dr. Heike Schmitt, Prof. Dr. Lars Nitschke and Prof. Dr. Thomas Winkler from the Division of Genetics reported that a particular protein called Siglec-H is responsible for the regulation of interferon secretion. Interferons are messenger substances of the immune system that are triggered in response to viral infection. If the protein Siglec-H was missing, the interferon secretion remained high, even if the infection had already subsided, causing an autoimmune disease similar to SLE.
Researchers now suspect that there are similar proteins to Siglec-H and thus comparable mechanisms which regulate interferon secretion and prevent the emergence of an autoimmune disease such as SLE. Strengthening interferon-inhibiting mechanisms through this group of proteins could therefore represent a novel therapeutic approach for SLE.
The study now published in the Journal of Experimental Medicine (doi: 10.1084/jem.20160189) was created in co-operation with the Division of Genetics and Division of Biochemistry at FAU, and the Department of Nephropathology at Universitätsklinikum Erlangen. It was also supported by the German Research Foundation in the framework of a Research Training Group (GK1660) and a Collaborative Research Centre (SFB1181).
Further information:
Prof. Dr. Lars Nitschke
Phone: +49 9131 8528453
lars.nitschke@fau.de