Cholesterol May Help Proteins Pair Up to Transmit Signals Across Cell Membranes
Simulations reveal new insights into the function of a protein involved in cancer metastasis and AIDS.
Cholesterol may act as a selective glue that binds proteins into paired structures that enable human cells to respond to outside signals, according to a new study in PLOS Computational Biology.
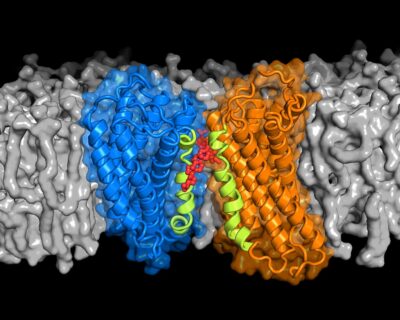
G protein coupled receptors (GPCRs) are a family of proteins found embedded in the outer membranes of eukaryotic cells. GPCRs sense signals outside of a cell, such as hormones or sugars, and transform them into a cellular response. To properly sense and respond to an external signal, evidence suggests, some GPCRs must pair up to form two-protein structures called dimers.
In the new study, Kristyna Pluhackova, Stefan Gahbauer and colleagues of the Computational Biology Group at the Friedrich-Alexander University of Erlangen-Nürnberg, Germany, used a GPCR known as chemokine receptor type 4 (CXCR4) to explore GPCR dimer formation at the molecular level. They ran more than 1,000 computer simulations to examine how other molecules that neighbor CXCR4 in the cell membrane, such as cholesterol, might affect the dimer formation.
The simulations revealed that CXCR4 requires cholesterol in order to pair up properly. Cholesterol molecules selectively “glue” specific regions of two CXCR4 molecules to each other, forming a structure that can sense and transmit external signals. Without enough cholesterol in the nearby membrane, CXCR4 proteins can still bind to each other, but these misshapen structures are likely unable to sense signals.
Normally, CXCR4 helps the human immune system function properly. However, this protein also plays a major role in metastatic breast and lung cancer, as well as in AIDS. Scientists also estimate that 40 percent of all prescription drugs act on GPCRs, in general. Therefore, understanding exactly how cholesterol affects GPCR dimer formation could open up new lanes in drug discovery, says study coordinator Rainer Böckmann.
Further information:
Prof. Dr. Rainer Böckmann
Tel.: 09131/85-25409
rainer.boeckmann@fau.de