Chemists break bonds in molecular nitrogen with calcium
New findings in fundamental research
Chemists all over the world are constantly searching for simple ways to make elemental nitrogen or N2 in the air available for chemical reactions. This is no easy task, as nitrogen is a particularly non-reactive gas with a triple bond, which is one of the strongest known chemical bonds. A research team at FAU has now demonstrated that calcium, a metal commonly found in nature, is able to break the highly-stable nitrogen bond and can do so at minus 60°C. This is significant for two reasons. On the one hand, the researchers at FAU have made a new discovery in terms of the bond-breaking capabilities of calcium, which had been largely disregarded in the past. On the other hand, their findings could form the basis for developing industrial processes in the future.
Nitrogen is one of the main components of air and is in unlimited supply. It is also used as an inert gas for protecting food due to its particularly low chemical reactivity and it can keep products such as part-baked rolls fresh for months. Plants also require nitrogen for growth. However, they cannot use nitrogen directly from the air. The greatest challenge lies in converting the highly-stable diatomic molecule N2 into useful chemicals. Two German chemists succeeded in doing so in the early 1900s when they developed the Haber-Bosch process, which converts N2 into ammonia (NH3). Whilst ammonia was originally used to manufacture explosives, today it is mostly used as a fertiliser. In the Haber-Bosch process, a transition metal catalyst triggers the chemical reaction. Conversion of highly-stable nitrogen into ammonia requires high pressures and high temperatures, which means Haber’s ‘bread from the air’ process requires large amounts of energy.
Chemists are looking for other methods of breaking the strong N≡N triple bond to simplify this and other processes. The team of researchers led by Prof. Dr. Sjoerd Harder, Chair of Inorganic and Organometallic Chemistry at FAU, have now successfully demonstrated that the main group element calcium is capable of achieving this feat. Calcium is a metal commonly found in nature mainly in limestone, which had been regarded in the past as not being capable of breaking strong chemical bonds. Unlike transition metals, which are often toxic, calcium is generally not capable of utilising d orbitals – a wave function with a specific symmetry that facilitates bond breaking reactions.
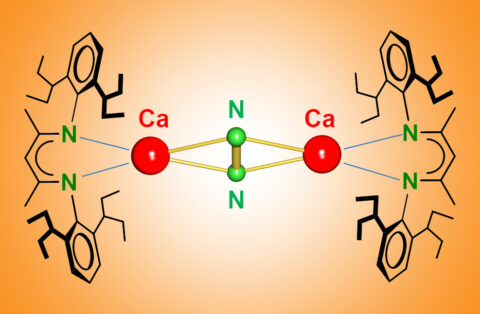
While searching for calcium atoms in the unusual oxidation level +I, the FAU researchers accidentally discovered that the metal reacts with nitrogen, which was only supposed to be used as an inert gas during the experiment. Harder and his team isolated a molecule that was trapped in the nitrogen between two calcium atoms and were able to continue the conversion to hydrazine. In contrast to nitrogen, which is extremely stable, hydrazine is used as highly-reactive rocket fuel. Working with theoretical chemists at the universities of Marburg in Germany and Nanjing in China, the FAU research team discovered that d orbitals do actually play a significant role in nitrogen activation with calcium. This controversial but significant discovery dispels the dogma that d orbitals are irrelevant for metals assigned to the main group in the periodic system.
Despite the fact that the process is neither catalytic nor economical, it provides new fundamental and important insights into bond breaking reactions with calcium. These findings will not only rewrite students’ textbooks, but could also contribute to the development of simplified industrial processes.
Further information:
https://science.sciencemag.org/content/371/6534/1125
Prof. Dr. Sjoerd Harder
Chair of Inorganic and Organometallic Chemistry
Phone: +49 9131 85 27350
sjoerd.harder@fau.de