Putting an end to rheumatoid arthritis?
Interdisciplinary research team unlocks the mechanism for inhibiting inflammation of the joints
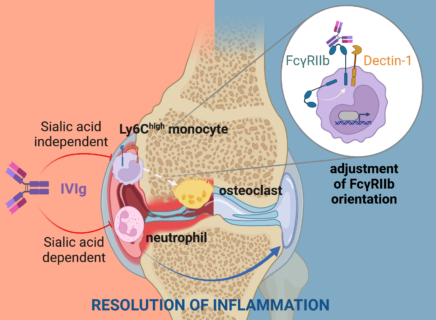
Immunoglobulin G antibodies (IgB) play an important role as drivers of inflammation in infectious diseases and autoimmune diseases. However, if the same immunoglobulin antibodies from the blood plasma of healthy donors are cleansed and injected into a patient’s bloodstream, they exhibit anti-inflammatory effects and have a positive effect on the immune system. The cause of this was unknown to a large extent up to now. An interdisciplinary team of researchers from Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) and the universities of Ulm and Würzburg led by Prof. Falk Nimmerjahn (Chair of Genetics at FAU) has now unlocked the mechanism that causes these intravenous immunoglobulin antibodies to resolve joint inflammation in rheumatoid arthritis. The researchers have published their findings in the journal Immunity.
The results indicate that antibodies from healthy donors, also known as intravenous immunglobulin antibodies, or IVIg for short, are able to suppress a central self-destructive process of rheumatoid arthritis – the degeneration and remodeling of bones and cartilage tissue in joints. “This bone degeneration caused by the inflammatory reaction leads to severe damage of the joints in patients of rheumatoid arthritis,” explains Prof. Nimmerjahn. “The results of the study now show for the first time how this process is suppressed on the molecular level by antibodies.” What surprised the interdisciplinary research team most of all was that molecules usually associated with fighting off pathogens such as bacteria and fungi play a central role for the anti-inflammatory effect of intravenous immunoglobulin antibodies. If these receptors were missing, the antibodies could no longer protect against inflammation and bone loss. These findings are of great importance for the development of new therapies for autoimmune diseases and inflammation triggered by cytokines and autoantibodies.
Collaboration with leading experts in the atomistic simulation of receptors and cell membranes (Prof. Rainer Böckmann, FAU), who benefited from the optimal environment of the Center for National High Performance Computing Erlangen (NHR@FAU), was essential for this interdisciplinary study. Collaboration with the world’s leading researchers in the field of high-resolution microscopy (super resolution microscopy) led by Prof. Markus Sauer (University of Würzburg) was equally as important. The research was carried out with funding from the DFG as part of CRC 1181 ( Checkpoints for Resolution of Inflammation; speaker Prof. Georg Schett, FAU).
Further information:
Prof. Dr. Falk Nimmerjahn
Chair of Genetics
falk.nimmerjahn@fau.de